Students must start practicing the questions from CBSE Sample Papers for Class 10 Science with Solutions Set 8 are designed as per the revised syllabus.
CBSE Sample Papers for Class 10 Science Set 8 with Solutions
Time : 3 Hr.
Max. Marks : 80
General Instructions:
- This question paper consists of 39 questions in 5 sections.
- Alt questions are compulsory. However, an internal choice is provided in some questions. A student is expected to attempt only one of these questions.
- Section A consists of 20 objective type questions carrying 1 mark each.
- Section B consists of 6 Very Short questions carrying 02 marks each. Answers to these questions should in the range of 30 to 50 words.
- Section C consists of 7 Short Answer type questions carrying 03 marks each. Answers to these questions should in the range of 50 to 80 words.
- Section D consists of 3 Long Answer type questions carrying 05 marks each. Answer to these questions should be in the range of 80 to 120 words.
- Section E consists of 3 source-based/case-based units of assessment of 04 marks each with sub-parts.
SECTION – A (20 Marks)
(Select and write one most appropriate option out of the four options given for each of the questions 1-20)
Question 1.
On heating ferrous sulphate (green in colour), Bhawna observed that it turns into a brown coloured substance which also releases foul smelling gases as shown in the figure given below. (1)

Help her to identify the type of reaction that occurred on heating ferrous sulphate:
(a) Decomposition reaction
(b) Displacement reaction
(c) Combination reaction
(d) None of these
Answer:
(a) Decomposition reaction
ExpLanation: A decomposition reaction is a reaction in which a single compound breaks
Question 2.
Which diagram dispLays the electrical, circuit for a home? (1)


Answer:

ExpLanation: Domestic electric give an idea of how power is supplied to homes using various types of switches. wires and circuits.
Question 3.
The following figure shows a type of movement in plants. (1)

(a) Hydrotropism
(b) Geotrapism
(c) Thigmotropism
(d) None of the above
Answer:
(c) Thigmotropism
Explanation: Thigmotropism or curvature movement is a plant movement in response to touch/contact. The region of contact has less auxin and the other side have more auxin, thus, shows more growth. This unequal distribution of auxin causes the tendril to coil over the support.
![]()
Question 4.
Given below is the picture of tiny pores present on the green parts of the plants that help in gaseous exchange, identify A, B, C and D in the given diagram: (1)

(a) A – Subsidiary cell, B – Epidermal cell, C – Stomatal pore, D – Guard cell
(b) A – Epidermal cell, B – Subsidiary cell, C – Stomatal pore, D – Guard cell
(c) A – Guard cell, B – Subsidiary cell, C – Stomatal pore, D – Epidermal cell
(d) A – Epidermal cell, B – Guard cell, C – Stomatal pore, D – Subsidiary cell
Answer:
(b) A-Epidermal cell, B-Subsidiary cell, $C$ Stomatal pore, D-Guard cell
Explanation: Structure of Stomata

Related Theory
Stomatal pore: These are tiny pores present on leaf surface and green parts of plants, that help in gaseous exchange, transpiration and photosynthesis.
Guard cell: Helps in opening and closing of srom atol aperture.
Subsidiary cells: These are the cells that surround guard cells and may provide mechanical support to guard cell that facilitates guard cell movements. They can also act as a reservoir for water and ions. down into two or more substances (elements or compounds). Most decomposition reactions required an input of energj in the form of heat, light or electricity.
Epidermal cell: Forms the outermost layer of plants. The epidermis and its waxy cuticle provide protection against mechanical injury, water loss, and infection.
Question 5.
Gulshan wants to write a balanced chemical equation for the following chemical reaction. (1)

Help him to choose the correct option:
(a) 3BaCl2 + Al2(SO4)3 → 3BaSO4 + 2AICl3
(b) BaCU + Al2(SO4)3 → BaSO4 + 2AICl3
(c) 3BaCl2 + Al2(SO4)3 → 3BaSO4 + AlCl3
(d) BaCl2 + Al2(SO4)3 → BaSO4 + AlCl3
Answer:
(a) 3BaCl2 + Al2(SO4)3 → 3BaSO4 + 2AICl3
Explanation: We can depict the number of atoms of elements in reactant side and product side in the equation:
3BaCl2 + Al2(SO4)3 → 3BaSO4 + 2AlCl3, as:
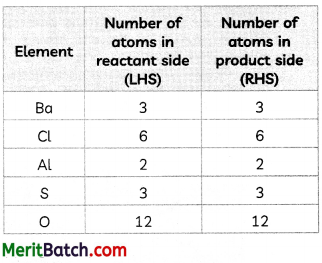
Question 6.
The following picture of on experimental set-up shows the response of roots towards water: (1)

This response of plant or plant parts towards water is known as:
(a) thigmotropism
(b) geotropism
(c) phototropism
(d) hydrotropism
Answer:
(d) Hydrotropism
Explanation: The movement of pI.ant in response to concentration of water is termed as hydrotropism.
Related Theory
- Thigmotropism: response to touch.
- Geotropism: response to gravity.
- Phototropism: response to light.
![]()
Question 7.
The following diagram shows the process of reproduction that occur in Plasmodium. (1)

Identify the type of reproduction:
(a) Budding
(b) Binary fission
(c) Spore formation
(d) Multiple fission
Answer:
(d) Multiple fission
ExpLanation: Multiple fission involves the ability of a cell to divide into several, cells during reproduction. Plasmodium divides by repeated division of cell to produce many daughter cells simuftaneously.
Related Theory
Budding: A mode of asexual reproduction in which a new organism develops from a small port of the parent’s body, which is termed as a bud. The bud which is formed, detaches from the parent’s body to develop into a new organism.
Binary fission: A type of asexual reproduction. usually found in prokaryotes like bacteria and a few single-celled eukaryotes. In this, there is a division of the parent cell into two new daughter cells.
Spore formation: Ir is a method of asexual reproduction, which involves formation of spores stored in sacs coiled sporangia. When these sporangia burst; minute single-celled. thin or thick walled structures called spores are dispersed and these develop into a new plant under suitable conditions.
Question 8.
Which of the following statements about magnetic lines of magnetic field is incorrect? (1)
(a) The north pole of a magnetic compass is assumed to point in the direction of the magnetic field at a point.
(b) Magnetic field lines are curled, closed lines.
(c) Zero field strength is represented by parallel and evenly spaced magnetic fteld lines.
(d) The degree of closeness of the field lines indicates the relative strength of the magnetic field.
Answer:
(c) Zero field strength ¡s represented by parallel and evenly spaced magnetic field lines.
Explanation: If magnetic field lines are parallel. in a given area of space, then that area has a homogeneous field strength.
![]()
Question 9.
The following picture shows a leaf which is tested with iodine solution, in an experiment – “Light is essential for photosynthesis”. (1)

Why does the uncovered part of the leaf turn blue black after addition of iodine solution?
(a) Starch reacts with iodine
(b) Chlorophyll reacts with iodine
(c) Sunlight causes colour change
(d) Chlorophyll reacts with starch
Answer:
(a) Starch reacts with iodine
Explanation: In the experiment – light is essential for photosynthesis”, a part of leaf was covered with black paper and the plant was then kept in sunlight. The uncovered part of the leaf performed photosynthesis in the presence of sunlight and produced starch. Therefore, when iodine is added, starch reacts with it to give a blue-black colour.
Caution
Students should know that the covered portion of the leaf could not get the sunlight as a result could not accumulate starch. Therefore, on addition of iodine, it does not turn blue-black.
Question 10.
Which of the following does not show how the environment may be harmed by the indiscriminate use of pesticides? (1)
(a) Overuse of DDT reduced the number of fish-eating bird species.
(b) Fish eating birds accumulated DDT as they moved down in the food chain.
(c) The formation of the egg shell was hampered.
(d) Due to the thinness of the shell it broke.
Answer:
(b) Fish eating birds accumulated DOT as they moved down in the food chain.
Explanation: Overuse of DDT reduced the number of fisheating bird species. Such birds accumulated DDT as they moved up the food chain. The formation of the egg shell was hampered. Due to the thinness of the shell and the weight of the bird during incubation, it broke. Consequently, their population declined.
Related Theory
The chemicals used to kill plant and animaL pests are known as pesticides. They ore toxic arid non-biodegradable.
![]()
Question 11.
Choose the hormone which is responsible for bending of plant shoot towards sunlight: (1)
(a) Auxin
(b) Gibberellin
(c) Cytokinin
(d) Ethylene
Answer:
(a) Auxin
Exptanation:The plant shoot bends towards sunlight, as, auxin is produced in the stem and it diffuses towards the shady area and accumulates there leading to elongation of cells in that part. This differential elongation causes bending of shoot. This is also termed as phototropism.
Question 12.
Although the sex of an organism is genetically determined, there are some organisms whose sex is not genetically determined. Example of this is: (1)
(a) Lizard
(b) Human
(c) Dog
(d) Snail
Answer:
(d) Snail
Explanation: In some organisms, sex is not genetically determined i.e., it is not determined by the genes inherited from the parents. The organisms in which sex ¡s not genetically determined are snails, turtles.
Related Theory
In snails, the sex determination occurs by using physical contact, the larva develops into male if it is in association with the female, whereas if alone it forms female.
In some other organisms, the sex of an individual is temperature-dependent. The temperature which the fertilised eggs ore kept will determine the sex of new-born. The temperature of the developing eggs decides whether the offspring will be mole or female. In turrles temperature above 33°C produces females and below 28°C produces males.
Question 13.
Light refraction on a mirror is depicted in the figure. (1)

What conclusion can be drawn from the figure?
(a) Angle of incidence and reflection at the point of reflection all lie on the same plane.
(b) Angle of incidence and normal at the point of reflection all lie on the same plane.
(c) Angle of incidence and reflection at the point of refraction all lie on the same plane.
(d) Angle of incidence, reflection and normal at the point of reflection all lie on the same plane.
Answer:
(d) Angle of incidence, reflection and normal at the point of reflection all lie on the same plane.
Explanation: The figure shows that angle of incidence, reflection, and normal at the point of reflection all lie on the same plane.
![]()
Question 14.
Seeta studied in her biology lecture that plants produce oxygen during the process of photosynthesis as a by-product and gives out oxygen during the day, whereas plants produce carbon dioxide during respiration and give out carbon dioxide during the night. Do you agree with this that all plants give out O2 during the day and CO2 during the night? (1)
(a) Yes
(b) No
(c) May be yes
(d) Data is insufficient
Answer:
(a) Yes
ExpLanation: No, don’t agree with this statement because all plants carry out respiration throughout the day and night i.e., 24 hours and thus, gives out CO2 during day as well as night. But during day, since sunlight is essential to carry out the process of photosynthesis, therefore, oxygen is released during daytime only.
Caution
Students should know that the process of gaseous exchange in plants takes place by diffusion depending on environmental conditions and plant requirements.
During day. rote of photosynthesis is more than that of respiration, CO2 generated during respiration is used in the process of photosynthesis.
Therefore. O2 release is the major event During night, photosynthesis does nor rake place due to absence of sunlight. therefore, CO2 is released.
Question 15.
Calculate the amount of charge passed through any area of cross section of the conductor when a current of 20 A flows through it for three minutes. (1)
(a) 36 C
(b) 1800 C
(b) 3600 C
(d) 180 C
Answer:
(b) 1800 C
ExpLanation: Given that
l = 20 A,
t = 3min = 3 x 60s = 180s
So,
Amount of charge Q passed through any area of cross-section is given by
or Q = l x t
Q = (20 x 180)As = 3600C
Caution
Students usually make mistake during calculations. They do not convert minutes into seconds which results ¡n wrong answer.
![]()
Question 16.
Which of the following defines the electric current?
(a) The amount of charge flowing through a particular area in unit time.
(b) One coulomb of charge flowing per second.
(c) The amount of current through a particular area in unit time.
(d) One coulomb of charge flowing per minute. (1)
Answer:
(a) The amount of charge flowing through a particular area munit time
Explanation: Electric current is defined as the amount of charge flowing through a particular area in unit time.
Caution
Students may get confused between option (a) and (b). Option (a) defines electric current and option (b) defines 1 ampere which is the unit of electric current.
Related Theory
One ampere ¡s constituted by the flow of one coulomb of charge per second
IA = 1 Cs1
Question Q. no 17 to 20 are Assertion – Reasoning based questions.
These consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true and R is not the correct explanation of A
(c) A is true but R is false
(d) A is false but R is true
Question 17.
Assertion (A): Hydrogen is not included in the activity series of metals.
Reason (R): Like other metals hydrogen can lose electrons to form positive ions. (1)
Answer:
(d) A is false but R is true.
Explanation: Hydrogen is included in the activity series of metals like other metals as hydrogen can also lose electrons to form positive ions.
Question 18.
Assertion (A): A reaction between a metal and nitric acid does not evolve hydrogen gas.
Reason (R): Nitric acid is a strong oxidising agent. (1)
Answer:
(a) Both A and R ore true and R is the correct explanation of A.
Explanation: A metal reacts with nitric acid. to form a metal nitrate, oxides of nitrogen and water and hydrogen gas is not formed, because nitric acid is a strong oxidising agent and it oxidises the hydrogen gas to water and nitric acid itself gets reduced to oxides to nitrogen like NO2, NO, etc.
M + 2HNO3 → MNO3 + NO2 + H2O (M = metaL)
Question 19.
Assertion (A): A geneticist crossed two plants and got 50% plants with white flowers and 50% with red flowers.
Reason (R): One plant is heterozygous for the trait and the other is homozygous recessive. (1)
Answer:
(a) Both A and R are true and R ¡s the correct explanation of A.
ExpLanation:
In the given cross, geneticists found 50% plants were with white flowers and 50% with red flowers. Let’s assume the red character to be recessive which can onlj be expressed in homozygous condition. Plant with white flowers is dominant. Because 50% population of the plant are with red flowers, so, it can be concluded that one of the parent plants must be heterozygous.
Let, the genotype of the heterozygous plant be Ww (W – white, w – red). W is dominant over w it appears phenotypically. The genotype of plant with red flowers is ww.
The cross can be shown as:

Related Theory
According to Mendel. when an allele passes from parent to offspring it segregates independently during the formation of gamete and expresses its phenotypic expression in the offspring without mixing with other alleles.
![]()
Question 20.
Assertion (A): The drooping of leaves in ‘touch me not’ plants occur due to smelling.
Reason (R): By changing the amount of water (turgor changes) in leaves, the plant cells change their shape. (1)
Answer:
(d) A is false but R is true.
Exptcincition: The leaves of Mimosa pudica (touch me not plant) shows movements when touched. When (eaves are touched, the stimulus reaches the base of leaf and the vacuoles of leaf cells toses water, which causes the Leaf to become flaccid. As a result, the leaf closes. This occurs due to changes in turgor pressure.
SECTION – B (12 Marks)
(Q. no. 21 to 26 are very short answer questions.)
Question 21.
Reema went to the market with her mother where she saw pickles kept in either glass or plastic jars and not in metal containers. She asked her mother why is it so. What could be the reason given by her mother? (2)
Answer:
Glass or plastic containers are far Less reactive to the contents inside them than metal containers. Pickles Contains some acids, oils. etc that reacts with metals and corrode them to produce toxic substonces.
Question 22.
How can you say that the difference in the molecular mass of any two adjacent homologues is 14 amu?
OR
Draw the electron dot structure of carbon dioxide. Identify the pair of electrons which forms covalent bond. Also state the number of double bonds in the structure. (2)
Answer:
The difference between the two successive homologues is of one carbon atom and two hydrogen atoms in terms of atoms in their molecules and differ by 14 amu in terms of molecular mass. This can be explained by an
example:
C4H8 and C5H10 are successive compounds and these two differ by – CH2
Atomic mass of carbon = 12µ
Atomic mass of hydrogen = 1µ
Molecular mass of – CH2 = (1 x 12) + (2 x 1)
= 14 µ

The shared pair of electrons is said to constitute o single covalent bond. So, there are 2 double bonds in CO2. The two oxygen atoms are bonded on either side with carbon atom by double bonds.
Related Theory
The shored pair of electrons is said to constitute a single covalent bond So. there are 2 double bands in CO2 The two oxygen atoms are bonded on either side with carbon atom by double bonds.
Question 23.
A student performed o cross between plants with purple flowers and plants with white flowers. Help him to complete the figure by finding ‘P’, ‘Q’, ‘R’ and ‘S’. Also write the genotypic ratio obtained in F2 generation. (2)

Answer:
The compIete figure is:

![]()
Question 24.
Saloni was stung by a wasp while playing in the garden and she started crying due to pain and irritation. Her mother immediately applied a coating of toothpaste on the affected area, which gave her an instant relief and then took her to a doctor. What does wasp sting contain and why did her mother apply toothpaste on the affected area?
OR
Sushma’s mother baked a coke, but to her surprise she found that the cake is hard and small in size. She thought and remembered that she forgot to add one important ingredient in her cake that wouLd have made the cake fluffy. Which is the ingredient that she forgot to add? Also write the chemical reaction. (2)
Answer:
Wasp sting contains formic acid (HCOOH) which cause pain and irritation. Because of the presence of mild base in the toothpaste which neutralises the effect of formic acid, it provides immediate relief to her. That is why her mother applied toothpaste.
Related Theory
Reaction between an acid and a base is catted as rieutrotization reaction which leads ro the formation of salt and water. Other examples include production of methanaic acid by stinging hair of nettle teaves that cause burning pain.
OR
Sushmo’s mother has forgotten to add baking powder (a mixture of sodium hydrogen carbonate and tortoric acid. While preparing the dough for the cake. The sodium hydrogen carbonate present in baking powder releases carbon dioxide on baking os depicted in the following reaction.
![]()
The carbon dioxide bubbles that evolve leave behind pores which make the cake soft and fluffy.
Question 25.
Neha was given some syrupy Liquid in a test tube by her teacher and she was told to dilute it with water. But by mistake, she added water quickly to the tube and the tube immediately cracked and the Liquid which spitted, produced busters on her skin. What could be the reason for this? (2)
Answer:
The syrupy liquid in the test tube could be conc. H2SO4 (sulphuric acid) or conc. HCl having great affinity for water and addition of water to an acid is a highly exothermic reaction, that results in excessive local heating of test tube and thus, cracking of the tube and spitting of acid that produced blisters.
Related Theory
The process of mixing on acid or base with water is coiled diLution, that resuLts in decrease in the concentration of ions (H3O+/OH–) per unit volume.
![]()
Question 26.
(A) A potential source is connected in parallel V by two wires of the same Length and area made of materials with resistivities of 1 and 2, respectively. What would the equivalent resistivity be if the length and area were the same?
(B) A source of potential V is connected in series with two wires that are of the same Length and oreo but made of different resistivity materials (1 and 2). What would the equivalent resistivity be if the area were the same? (2)
Answer:
Weknowthat, R = p \(\frac { l }{ A }\)
For the same Length and area of cross-section, resistance is directly proportional, to resistivity (p).
i.e., R ∝ p
(A) EquivaLent resistance is parallel connection of resistance is

(B) For series combination equivatent resistance is:
Rs = R1 + R2
As R ∝ p
So, Ps = P1 + P2
SECTION – C (21 Marks)
(Q.no. 27 to 33 are short answer questions.)
Question 27.
Sunita and Rajesh shifted to their new house and decided to get it white-washed. Sunita observed that the workers dissolved some white powdery material into water and the mixing resulted in a vigorous reaction and then they left it for some time. They then applied the white suspension obtained to the walls. In the beginning the wall looked pale white and watery but after two-three days the walls become shiny bright white in colour.
Answer the following questions:
(A) Name the white powdery substance and write its chemical reaction with water.
(B) Is it an endothermic or exothermic reaction?
(C) Why the walls turned shiny bright white • colour after two-three days?
OR
Reshma heated green coloured crystals of ferrous sulphate in a test tube, that resulted in the formation of brownish black coloured residue accompanied with a characteristic odour of burning sulphur as shown in the picture given below.

Answer the following questions:
(A) Name the brown coloured substance formed on heating and the gases that evolved along with it.
(B) Write the type of reaction that occurred above and why it is called so?
(C) Give reason for the change in the colour of the green crystals. (3)
Answer:
(A) The white powdery substance is calcium oxide (quicklime) – CaO. Quicklime reacts with water to form calcium hydroxide (slaked lime):
CaO(s) + H2O(l) —> Ca(OH)2(aq)
(B) The above reaction is an exothermic reaction as during this reaction a lot of heat is produced.
(C) During whitewash, Calcium hydroxide is applied to the walls and it reacts slowly with the carbon dioxide in air to form a thin layer of calcium carbonate on the walls. Calcium carbonate is formed after two to three days of white washing and gives a shiny finish to the walls.
Ca(OH)2(aq) + CO2(g) -> CaCO3(s) + H2O(l)
OR
(A) The brown coloured substance formed on heating ferrous sulphate is ferric chloride (Fe2O3) and the gases that evolved are sulphur dioxide (SO2) and sulphur trioxide (SO3).
(B) This is a type of decomposition reaction because in this reaction a single reactant breaks down to give simpler products.
(C) Ferrous sulphate crystals (FeSO4, 7H2O) lose water (water of crystallization) when heated and the green colour of the crystal changes to brownish black. It then decomposes to ferric oxide (Fe2O3), sulphur dioxide (SO2) and sulphur trioxide (SO3).
![]()
Question 28.
Siya was amazed to know that in plants, there are tiny pores called stomata present on the epidermis of leaves and other green parts of the plant. These stomatal pores help in gaseous exchange and this opening is in turn guarded by specialized kidney shaped cells called guard cells. But she was unable to understand how these guard cells open and close. Help Siya by explaining her how the guard cells regulate the opening and closing of stomata. (3)
Answer:
The stomatal aperture is guarded by two bean-shaped/kidney-shaped guard cells. These guard cells regulate the opening and closing of stomata by the osmosis process. When water enters inside the guard cells, they swell up and due to their curved surface, stomata open. Whereas, when water moves out, and it Leads to shrinking of the guard cells to become flaccid, this causes the stomata to close

Question 29.
(A) Teacher explained in the class that metals loose electrons to form cations and non-metals gain electrons to form anions. But carbon can neither form C4+ cations nor C4- anions, and forms covalent bonds. Why?
(B) Give reason why covalent compounds are bad conductors of electricity. (3)
Answer:
(A) Carbon atom is tetravalent, but it cannot gain or loose electrons because of the following reasons:
(i) If it gains 4 electrons, it will have negative charge (C4-), and it becomes difficult for 6 protons to hold on to 10 electrons and eventually the atom becomes unstable.
(ii) If it loses 4 electrons, it will require a lot of energy to lose them, which it can’t afford and as a result it becomes unstable again.
(B) Covalent bonds are formed by sharing of electrons and there is no transfer of electrons or involvement of ions. Because ions are responsible for conducting electricity and covalent compounds do not have free ions. Therefore, they are bad conductors of electricity. For example: CCl4, CH4.
![]()
Question 30.
In a country like India, the rate of female foeticide is quite high and always female is held responsible for giving birth to a female child. Reema was against this thought and practice, she raised her voice and started a campaign to create awareness among people. Help her to explain this. (3)
Answer:
The females are homogametic and produces only one type of gamete carrying only X chromosome, whereas males are heterogametic producing two types of gametes – half carrying X chromosome and the other carrying Y chromosome. Therefore, if a sperm carrying X chromosome fuses with the egg, it results in a female offspring, and if a sperm carrying a Y chromosome fuses with the egg, it results in a male offspring. Therefore, a father will be responsible for the sex of a child.
Sex determination in Human beings

Question 31.
Draw a neat and well labelled diagram of human alimentary canal. Name and label the following:
(A) Part carrying the food to the stomach through peristaltic movements.
(B) Organ producing a substance responsible for emulsification of fats.
(C) Organ secreting trypsin enzyme. (3)
Answer:
(A) Oesophagus
(B) Gall bladder
(C) Pancreas

Question 32.
A convex mirror is used in a car having a focal length of 300 cm. If a cycle is located at 100 cm from this mirror, find the position, nature and magnification of the image formed in the mirror. (3)
Answer:
We know,
Focal length ‘f’ = 300cm
So, object distance (u) = -100cm
Since the object (cycle) is placed to the left of the mirror, it is always taken with a negative sign.
\(\frac { 1 }{ f }\) = \(\frac { 1 }{ v }\) + \(\frac { 1 }{ u }\)
\(\frac { 1 }{ v }\) = \(\frac { 1 }{ f }\) – \(\frac { 1 }{ u }\)
\(\frac { 1 }{ v }\) = \(\frac { 1 }{ 300 }\) – (-\(\frac { 1 }{ 100 }\))
\(\frac { 1 }{ v }\) = \(\frac { 1 }{ 300 }\) + (\(\frac { 1 }{ 100 }\))
\(\frac { 1 }{ v }\) = \(\frac { 4 }{ 300 }\)
v = \(\frac { 300 }{ 4 }\)
v = 75cm
magnification ‘m’ = – \(\frac { v }{ u }\) = \(\frac { -75 }{ -100 }\)
m = 0.75
Therefore, the position of the image is 75cm behind the mirror and its nature is virtual and erect as it is formed behind convex mirror.
![]()
Question 33.
Draw a ray diagram for the following cases:
(A) When a ray of light passing through centre of curvature of a convex mirror is incident on it.
(B) A ray of light parallel to principal axis is incident on concave mirror.
(C) A ray of light passing through focus of convex mirror is incident on it.
OR
The picture demonstrates how the prism disperses the white light.

What colour will the letters P, Q, and R have? Also state the reason. (3)
Answer:


P represents violet.
Q represents green.
R represents red.
We are aware that waves with longer wavelengths deviate less, and vice versa. Therefore, the red wilL deviate the most while the violet colour the least. As a result, the upper part of the spectrum will be red, green in the middle and the lower part will be violet.

SECTION – D (15 MARKS)
(Q.no. 34 to 36 are tong answer questions.)
Question 34.
(A) Pratyush was studying about extractions of metals. He came to know about different new scientific terms. Help him to define the following: (3)
- Minerals
- Ores
- Gangue
(B) Write any two properties of titanium that makes it useful.
OR
(A) Iron is removed from the iron sulphate aqueous solution by an element “X.” If the element “X” is treated with aqueous solutions of copper sulphate, zinc sulphate, and silver nitrate, list your observations.
(B) Rapid effervescence is produced when hydrochloric acid is used to treat ore. Name the type of ore with one example.
(C)
- Aluminum, magnesium, and sodium oxides cannot be converted to their corresponding metals by carbon. Why?
- In the reactivity series, where are these metals located? How do these metals get their ores into metals?
- Use an illustration to describe the extraction process and the associated chemical equations. (5)
Answer:
(A)
(i) Minerals: These are the elements or compounds which occur naturally in the earth crust. These are combined state of metals with non-metals.
(ii) Ores: Minerals at some places, contain a very high percentage of a particular metal and the metal can be profitably and conveniently extracted from it. These minerals are called ores.
(iii) Gangue: The ores that are mined from the earth are usually contaminated with large amounts of impurities such as soil, sand, etc., called gangue.
(B) Two properties that makes titanium so useful are:
- It is stronger and light in weight. It is used to make war equipment.
- It remains unaffected by corrosion even if kept open for a very longer period of time.
OR
(A) X is more reactive than iron because it ousts iron from its salt solution. As both are less reactive than iron, it will also replace copper from copper sulphate and silver from silver nitrate. X may be more or less reactive than zinc because zinc is more reactive than iron.
(B) The ore produces rapid effervescence when treated with diluted hydrochloric acid; therefore, it must be a carbonate ore. A significant zinc carbonate (ZnCO3) ore is calamine.
(C)
(i) Sodium, magnesium, and aluminium highly reactive metals and have a higher affinity for oxygen than carbon does. Therefore, carbon cannot convert sodium, magnesium, or aluminium oxides to their corresponding metals.
(ii) The reactivity series places these metals at the top. Electrolytic reduction of their molten chlorides or oxides is used to extract the highly reactive metals like Na, Mg, Al, and others.
(iii) Electric current runs through the molten state to produce electrolytic reduction. At the cathode, metal is deposited.
NaCl ⇌ Na+ + Cl–
At cathode: Na+ + e–
Na At anode: 2CI– -> CI2 + 2e–
![]()
Question 35.
(A) When from earth we see sky, it appears
in different colours like blue, white, yellow, grey, etc. but for astronauts in space sky only appear to be dark. Explain the reason for this.
(B) Illustrate with three examples how colloidal particles show Tyndall effect.
OR
(A) How did Newton demonstrate the presence of seven colours in the sun’s white light?
(B) He used two identical glass prisms. Create a ray diagram to depict the path of light when two identical glass prisms are placed next to each other in an inverted position and a narrow white light beam is allowed to fall obliquely on one of the first prism’s focus.
(C) List two pre-requisites for seeing a rainbow. (5)
Answer:
(A) in space, there is no atmosphere, as a result there are no particles to disperse light coming from the Sun. Therefore, the light coming from the sky does not scatter. The light has to scatter for the sky to appear bright, so that we can see the sky clearly.
(B) Colloidal particles show Tyndall effect as they scatter light.
The examples are as follows:
- When a fine beam of sunlight enters a smoke-filled room, smoke particles become visible due to scattering of light by these particles.
- When a ray of sunlight passes through the canopy of a dense forest, tiny water droplets in the air scatter light.
- Milk is a colloid containing globules of fat and proteins. The light gets scattered when a beam of light is projected towards it.
OR
(A) The first person to obtain the spectrum of white light using a glass prism was Newton. The step was to align a second, identical prism with the first prism inverted. As a result, all the colours of the white light were able to pass through the second prism and combine to form a white light that emerged from its opposite side. This Led him to think that white light was made up of various colours.
(B)

(C) There are two prerequisites that must be met in order to see a rainbow:
- The Sun must be behind the observer.
- A rainbow should be visible through a waterfall or water feature or after it has rained.
Question 36.
(A) In a horse-shoe magnet, a current carrying conductor is placed perpendicular to the magnetic field.

The conductor is displaced upward. What will happen to the displacement of the conductor if:
- current in the conductor is increased.
- a horse-shoe magnet is replaced by another stronger horse-shoe magnet.
- the length of the conductor is increased.
(B) Which device can be used to maintain a potential difference between the ends of a conductor? Explain the process by which this device does so. (5)
Answer:
(A)
(i) The force acting on a current carrying conductor placed perpendicular to a magnetic field increases with the increase in the current flowing through a conductor. Thus, the displacement of the conductor will increase the current in the conductor is incread.
(ii) When a horse-shoe magnet is rei laced by a stronger magnet, then magnetic field increases. Since, force acting a conductor increases with the increase in the magnetic field, therefore, the displacement of the conductor will increase.
(iii) Since, the force acting on the conductor increases with the increase in the length of the conductor, therefore, the displacement of the conductor will increase.
(B) The device that can be used to maintain a potential difference between the ends of a conductor is a cell or a battery. The chemical reaction within a cell generates the potential difference across the terminals of the cell, even when no current is drawn from it. When it is connected to a conductor, it produces electric current and, maintain the potential difference across the ends of the conductor.
OR
(A) The magnetic force that a current-carrying wire feels when it is exposed to a magnetic field depends on the conductor’s
- current flow,
- magnetic field strength,
- length, and
- angle between the element of Length and the magnetic field.
(B) When the direction of the current is perpendicular to the direction of the magnetic field, the force experienced by a conductor carrying current when it is in a magnetic field is greatest.
(C) The Fleming left hand rule is used to determine the direction of motion of a conductor placed in a magnetic field.
(D)
- When the magnetic field’s direction is changed, the force acting on the conductor will now move from left to right.
- If the current is flowing in the opposite direction, the force acting on the conductor will move from left to right.
SECTION – E (12 Marks)
(Q.no. 37 to 39 are case – based/’data -based questions with 2 to 3 short sub – parts. Internal choice is provided in one of these sub-parts.)
Question 37.
Reha connected two or more resistances in such a way that the same potential difference gets applied to each of them. This kind of connection is said to be parallel. When she joined two or more resistances in parallel to one another, then the same current gets additional paths to flow and the overall resistance decreases. The equivalent resistance is given by (4)
![]()
(A) As shown, three resistances are connected.

Calculate the equivalent resistance between points A and B.
(B) When a 12m wire is divided into three equal pieces and its ends are then twisted together, find the resulting equivalent resistance.
OR
(B) If three resistances, 3Ω, 7Ω and 9Ω are connected in parallel, then what will be the equivalent resistance.
Answer:
(A) The equivalent resistance in the parallel combination is lower than the individual resistance’s lowest value.
All the three resistors (6Ω, 3Ω and 1Ω) are connected in parallel.

(B) Resistance of each piece = \(\frac { 12 }{ 3 }\) = 4Ω
When the end pieces of the three pieces are twisted together, the connection becomes parallel. And the equivalent resistance is calculated as:

OR
(B) Given that:
R1 = 3Ω
R2 = 7Ω
R3 = 9Ω
All the three resistors are in parallel arrangement,
So,

Question 38.
Neha visited Mughal Garden as shown in the picture, she felt so happy and amazed to see different types of flowers having different colours and fragrances. She told her teacher that flowers beautify our environment and refreshes our mood and we should plant more and more flowering plants to enjoy this beauty.

Her teacher told her that flowering plants are called angiosperms and they not only beautify our surroundings, but also form an important part of our ecosystem. (4)
(A) Where is the male and female gamete formed in flowering plant?
(B) Which part of the flower becomes seed after fertilization?
(C) Identify the parts Labelled as A, B, and C.

What do they constitute ?
(C) In which of the following category would you classify a Hibiscus flower? Explain. Give one example of unisexual flower. Explain sterile flowers.
Answer:
(A) Male gamete is found in anther and female gamete is found in ovary
(B) After fertilization, the ovules become the seeds, and the ovary wall becomes the fruit.
(C) A – Stigma, B – Style, C – Ovary Stigma, style and ovary constitute the female reproductive part of the flower – carpel/pistil.
Related Theory
Stigma – Sticky pollen-receptive part ofpistil
Style – The stalk of the pistil through which the pollen tube grows.
Ovary – Contains the ovules and it later develops into fruit.
OR
(C) Hibiscus has both male and female reproductive organs as stamens and pistil, therefore classified as bisexual flower. Unisexual flowers are those which have either male or female reproductive organ, such as in Papaya.
When both sexes i.e., male and female reproductive parts are absent in a flower or are non-functional, such flowers are called sterile.
![]()
Question 39.
On a site visit, Raj saw the poor condition of a river due to plastic pollution and overuse of pesticides and fertilizers as shown in the picture given below. India, is facing the problem of contamination of water, overuse of resources and plastic pollution. Nowadays a campaign has also started to avoid single use plastics. Efforts should be made to develop environmental-friendly substitutes and effective methods of safe plastic disposal. In agricultural practices also, there is excessive use of pesticides and fertilizers. These pesticides are absorbed by plants from the soil and from the water bodies, these pesticides are taken up by aquatic animals and plants, which severely affects them. These chemicals get accumulated in the bodies of plants, animals and even human beings. (4)

(A) If a river is found to be contaminated with coliform bacteria. Mixing of which pollutant in water is responsible for this?
(B) In comparison to biopesticides, chemical pesticides are more dangerous. Why?
(C) What is the term used for accumulation of non-biodegradable pesticides in the food chain in increasing amount at each higher trophic level? Explain.
OR
(C) Single use plastics should be avoided. Why? Name one environment friendly substitute for plastics.
Answer:
(A) Human faecal matter is responsiblee. If contains coliform bacteria which are present in small intestine, whereas bacteria is not present in industrial wastes, pesticides and fertilizers.
(B) Chemical pesticides are non-specific, hazardous, expensive and cause pollution.
(C) Biomagnification it is the accumulation of non-biodegradable pesticides such as DDT, industrial chemicals, radioactive substances in the food chain in increasing amount at each higher trophic level. Humans are at the top of the food chain; therefore, maximum concentration of these chemicals occur in them.
OR
(C) Non-biodegradable substances are those that cannot be broken down into simpler compounds by micro-organisms. So, these substances accumulate in the environment and cause pollution and severe health hazards. Resusable cloth bag is an environment friendly substitute for plastics.
