Heat and Temperature HC Verma Concepts of Physics Solutions
Heat and Temperature HC Verma Concepts of Physics Solutions Chapter 23
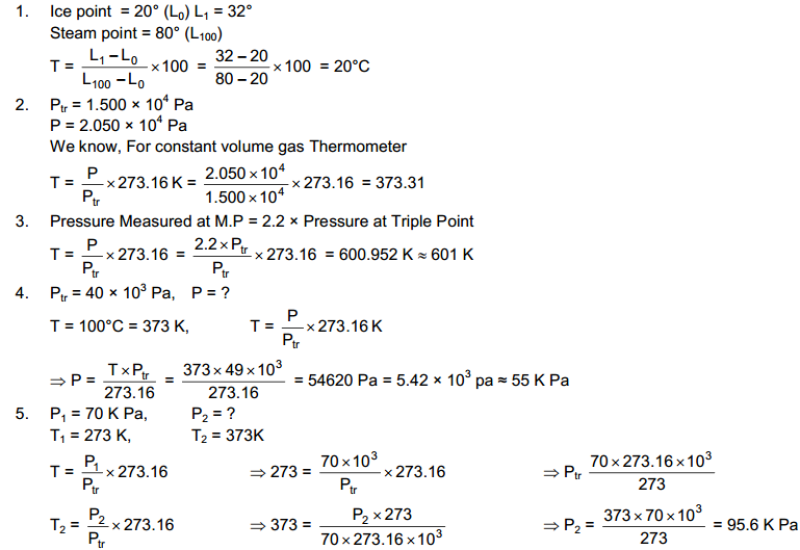
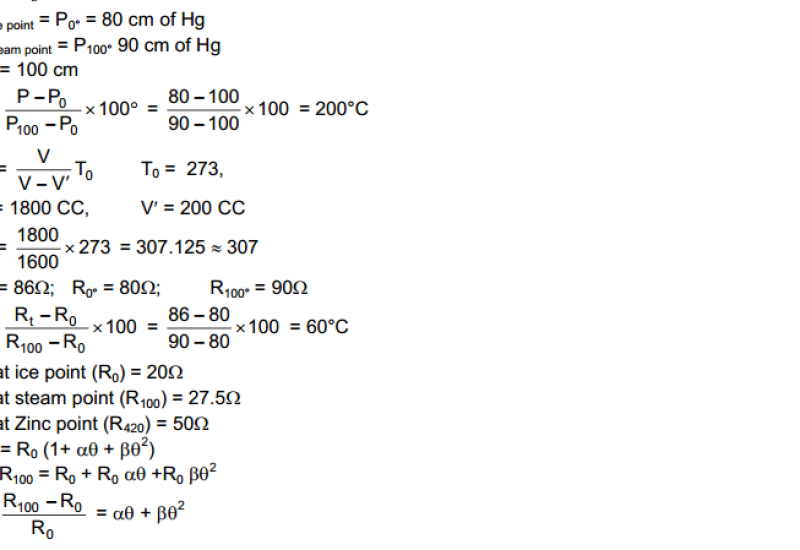
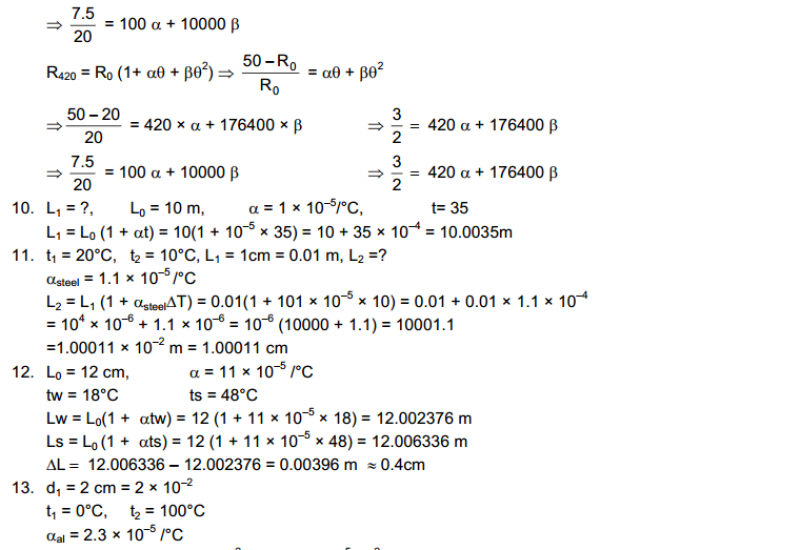
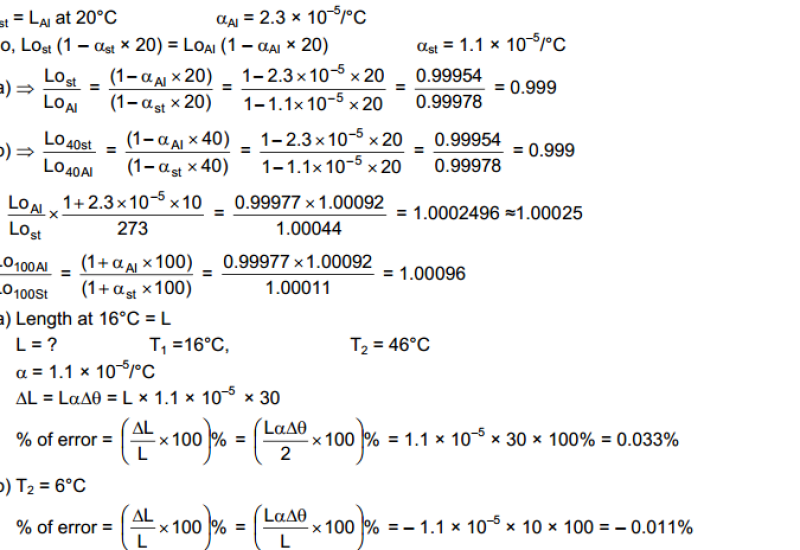

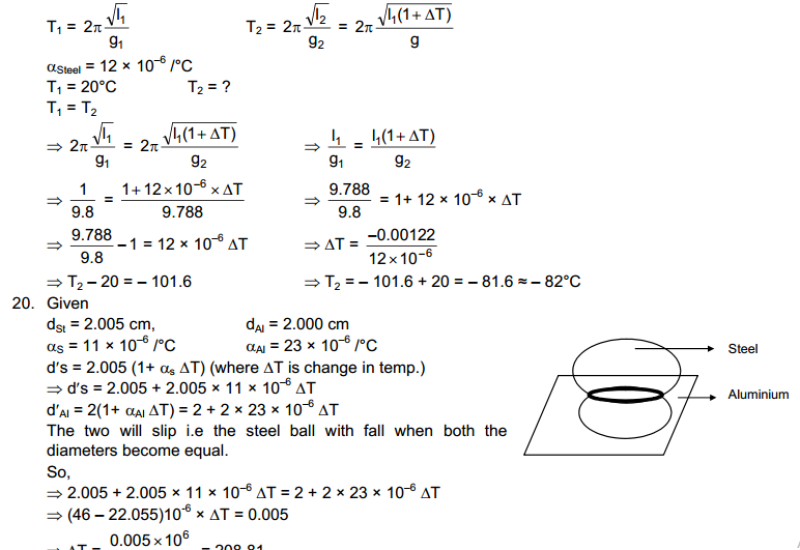



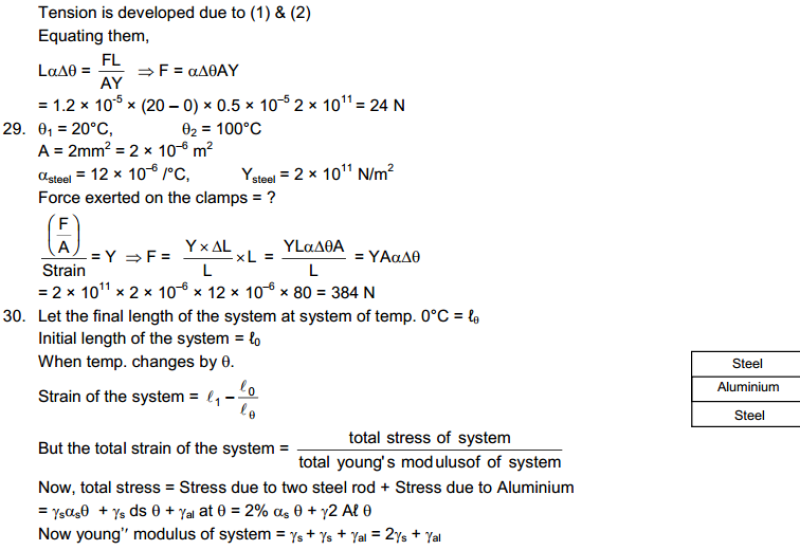
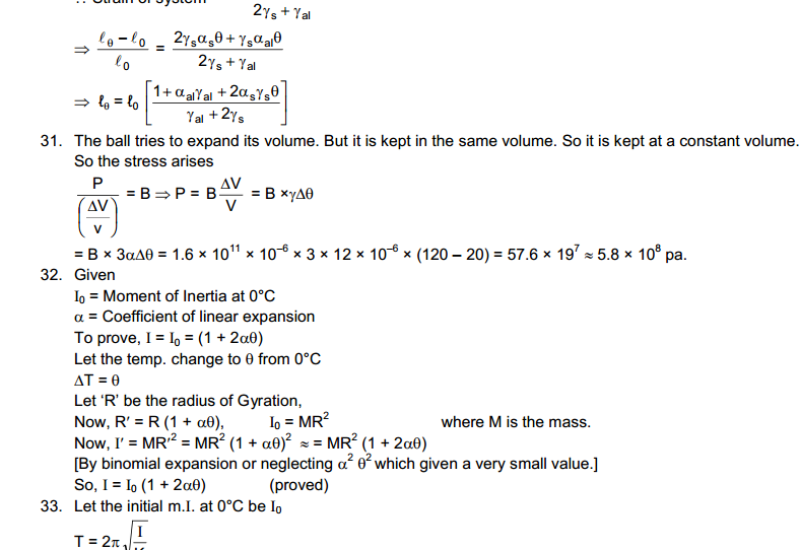
Hot and Cold Bodies:
When we rub our hands for some time, they become warm. When a block slides on a rough surface, it becomes warm. Press against a rapidly spinning wheel. The wheel slows down and becomes warm. While going on a bicycle, touch the road with your shoe. The bicycle slows down and the shoe becomes warm. When two vehicle;; collide with each other during an accident, they become very hot. When an aeroplane crashes, it becomes so hot that it catches fire.
In each of these examples, mechanical energy is lost and the bodies in question become hot. Where does the mechanical energy vanish? It goes into the internal energy of the bodies. We conclude that the cold bodies absorb energy to become hot. In other words, a hot body has more internal energy than an otherwise identical cold body.
When a hot body is kept in contact with a cold body, the cold body warms up and the hot body cools down. The internal energy of the hot body decreases and the internal energy of the cold body increases. Thus, energy is transferred from the hot body to the cold body when they are placed in contact. Notice that no mechanical work is done during this transfer of energy (neglect any change in volume of the body).
This is because there are no displacements involved. This is different from the case when we lift a ball vertically and the energy of the ball-earth system increases or when a compressed spring relaxes and a block attached to its end speeds up. In the case of lifting the ball, we do some work on the ball and the energy is increased by that amount. In the case of spring-block example, the spring does some work and the kinetic energy of the block increases.
The transfer of energy from a hot body to a cold body is a non-mechanical process. The energy that is transferred from one body to the other, without any mechanical work involved, is called heat.
Zeroth Law of Thermodynamics:
Two bodies are said to be in thermal equilibrium if no heat transfer of heat takes olace when they are placed in contact. we can now state the Zeroth law of thermodynamics as follows:
If Two Bodies X and Y are in thermal equilibrium and X and Z are also in thermal equilibrium then Y and Z are also in Thermal Equilibrium.
It is a matter of observation and experience that is described in the zeroth law. It should not be taken as obvious. For example, if two persons A and B know each other and A and C know each other, it is not necessary that B and C know each other.
The Zeroth Law of Thermodynamics allow us to introduce the concept of temperature to measure the hotness or coldness of a body. All Bodies in Thermal Equilibrium are assigned equal temperature. A hotter body is assigned a higher temperature than a colder body. Thus the temperature of Two Bodies decide the direction of heat flow when two bodies are put in contact. Heat Flows from the body at Higher Temperature to Lower Temperature.
We now define the property of thermal systems that determines whether or not they are in thermal equilibrium as the temperature: Two systems that are in thermal equilibrium with each other must have the same temperatures. Thermal equilibrium is the state in which there is no net interchange of thermal energy between bodies. In practice, temperature is measured by assigning numbers to systems we designate as cold or hot and by using thermal equilibrium.
The everyday scale of temperature is the Celsius scale, named for Swedish astronomer Anders Celsius (1701-1744), who designed the first temperature scale separated by one hundred degrees of temperature in 1742. As defined today, the Celsius temperature scale has its zero at the triple point of water (where water, ice, and water vapor are at equilibrium in a vacuum), a temperature negligibly different from the freezing point of water on Earth’s surface, and sets 100 °C as the boiling temperature of water at sea level. A degree Celsius (°C) is one-one hundredth of the interval between these two points. In this Celsius scale, ice melts at 0 °C, a cool day might have a temperature of 10°C, room temperature is about 20 °C, a heat wave would have temperatures around 30 to 40 °C, and human body temperature is 37 °C (skin temperature is about 35 °C).
The absolute scale, or Kelvin scale, is named for William Thomson, Lord Kelvin (1824-1907), who contributed mightily to the understanding of thermal systems. He was the first person to realize that there is an absolute minimum temperature at -273 °C, which he proposed in 1851. The kelvin (K) is equal in size to the degree Celsius. There is no “degree” associated with the name of the unit in the Kelvin scale. The zero of the absolute scale, absolute zero, is at 0 K = -273.15 °C. Thus, 0 °C is at a temperature of 273.15 K.
Constant Volume Gas Thermometer
A gas enclosed in a conductor has a definite volume and a definite pressure in any given state. If the volume is kept constant, the pressure of a gas increases monotonically with increase in temperature. This Property of a gas may be used to construct a thermometer.
The Platinum resistance Thermometer works on the principle of Wheatstone Bridge used to measure resistance.
The Temperature of the ice point on the ideal gas scale is 273.15 K and of the steam point is 373.15 K. The Interval between the two is 100 K. The Centigrade scale discussed earlier has 0 degree centigrade for the ice point and 100 degree centigrade for the steam point.
HC Verma Concepts of Physics NCERT Solutions Homepage RD Sharma Solutions
More Resources for CBSE Class 12: